

Conductivity measurement with a conductivity meter
Whether it is the cleaning of a filling system in a dairy or the protection of a cooling water system in a power plant, the correct operation of these processes depends, among other things, on the conductivity value. With our article, you will learn the theoretical basics of water conductivity, which will help you to improve the accuracy of your conductivity measurements.
Electrical conductivity (conductivity) - what is it?
Conductivity (ɣ) expresses how well a material conducts electricity. The electrical conductivity of water (known as the electrical conductivity of water or conductance of water) can therefore be defined as the ability of water to conduct electricity.
Ions or electrons? The resistance of water
In the case of metals, it is the movement of electrons that causes current to flow. In aqueous solutions, the transport of charge is taken over by ions, formed during the dissolution of salts, acids or bases. Conductivity depends on the number of ions present - the more of these molecules present in a liquid, the better it conducts electricity.
Conductivity measurement
The instrument generates an electrical voltage in the solution to be measured. A current then flows through it, the value of which depends on the conductivity. Depending on the method or application, the instrument either (1) maintains a constant voltage signal and records the change in electric current, or (2) maintains a constant current value and evaluates the change in voltage
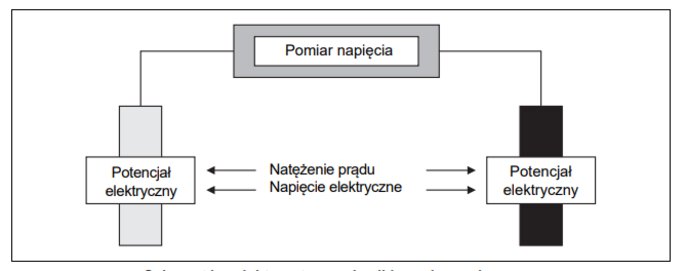
Conductivity measurement - schematic diagram
Ohm's law and the conductivity of water
Both methods are based on Ohm's law. At a constant voltage, the current increases in proportion to the conductance. In contrast, at constant current, voltage decreases as conductance increases.
It follows from Ohm's law that conductivity measurements are actually about resistance measurements. Therefore, the value of conductance I/U is obtained from the inverse of the value of resistance.
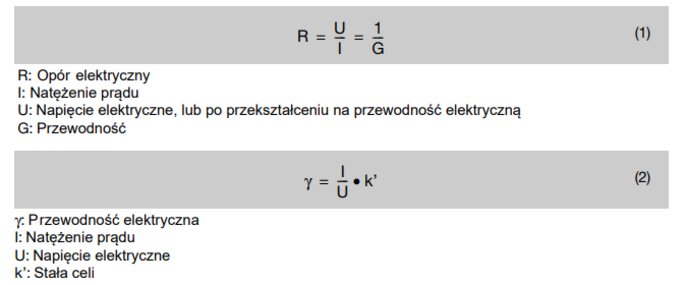
Ohm law
Polarisation and electrical conductivity
The electric current between the electrodes depends on the movement of ions in the solution being measured. During the conductivity measurement, ions move towards the electrode that is oppositely charged at any given time. Each ion that reaches one of the electrode surfaces aligns part of the voltage between the electrodes. Since it is no longer mobile, it blocks the flow of current.
This effect (polarisation) can be balanced by an alternating voltage. Due to the continuous reversal of polarisation, only a small number of ions reach the electrodes, and only for a short time. The more ions the solution contains, i.e. the higher its conductivity, the higher the frequency the device must use to prevent polarisation of the electrodes.
Electrolytic conductivity - what does it depend on?
The conductivity of water is influenced by:
-
the degree of impurity - the more impure the liquid, the greater the conductivity. Pure water is almost non-conductive.
-
temperature - the temperature of the solution must be known for the measurement to be correct, because conductivity is highly temperature dependent. A 1°C increase in temperature results in an increase in ion mobility and therefore a higher conductivity of about 2% for tap water and about 6% for high-purity water. The temperature can be measured automatically using a temperature sensor (e.g. Pt 100 or Pt 1000) or set manually by the user (temperature compensation).
Measuring targets
2-electrode conductivity cell
Two-electrode measuring cells are the simplest construction of a conductivity measuring cell. They consist of two electrodes and a sheath that fixes both electrodes. A constant alternating voltage is applied between the electrodes. The measuring signal is the current flowing through the solution to be measured.
Two-electrode measuring cells are fully sufficient for industrial measurements. The type, constant and nature of the electrode surface depends on the specific application for which the cell is to be used.
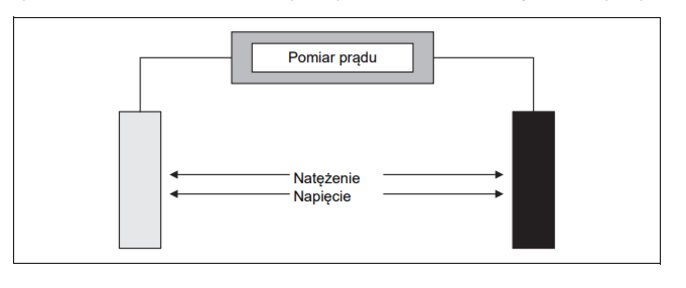
2-electrode measuring cells
4-electrode conductivity cell
There are two pairs of electrodes on the 4-electrode measuring targets. One pair measures the current, the other the voltage applied to the solution to be measured. They have the advantage of low sensitivity to the effects of resistance interference caused, for example, by long connection wires, impurities or polarisation. This leads to low readings because they reduce the voltage that the electrodes apply to the measured conductivity. The second pair of electrodes determines the voltage on the measured solution conductivity. The device can take into account the interference resistance by means of an electronic adjustment based on the measured current/voltage values for the two electrode pairs.
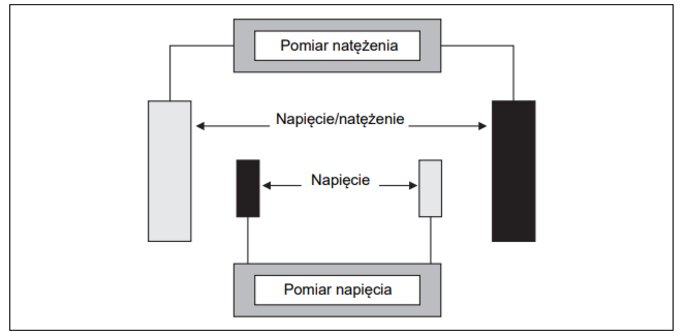
4-electrode measuring cells
Applications of conductivity
Conductometry is used in a variety of applications. Conductivity measurements are important for industry and environmental protection, among others. Depending on the application, the measurement can take place in the laboratory, on site with a hand-held instrument or continuously, e.g. in a process environment.
Electrolytic conductivity measurement is carried out in places such as:
-
sewage treatment plants
-
zinc works
-
beverage bottling plants
-
pharmaceutical production
-
power plants
-
desalination plants.
Conductivity is an important tool for monitoring different types of water (including pure distilled or deionized water, drinking water, natural water, process water or chemical water quality) and other solvents like total dissolved solids. Use a sensor with a low cell constant (0.01-0.1 cm-1) for low conductivity measurements and a sensor with a high cell constant (0.5-1.0 cm-1) for medium and high conductivity measurements.

Wastewater treatment plant - one of the places where conductivity is measured
JUMO conductivity sensors
The choice of the right conductivity sensor is a decisive factor in obtaining accurate and reliable results. At JUMO, we have high-end water conductivity sensors - with 2 or 4 electrodes and with digital interfaces such as JUMO digiLine or IO-Link. Check out our conductivity meters and see for yourself!
About the Author





